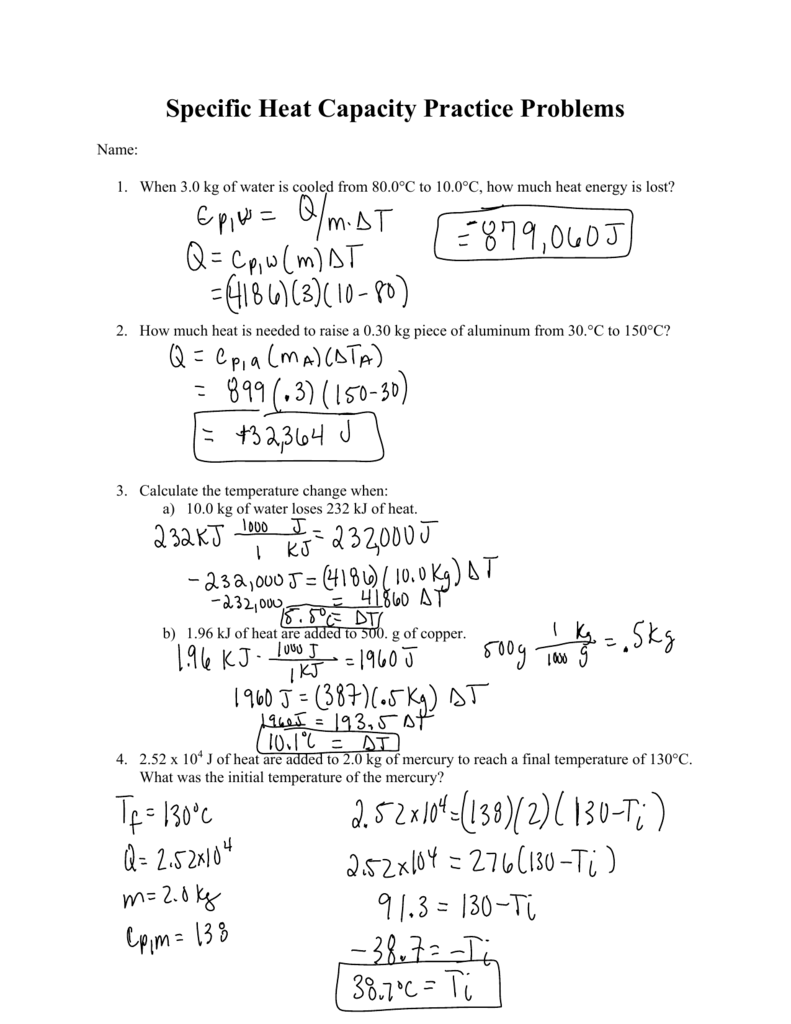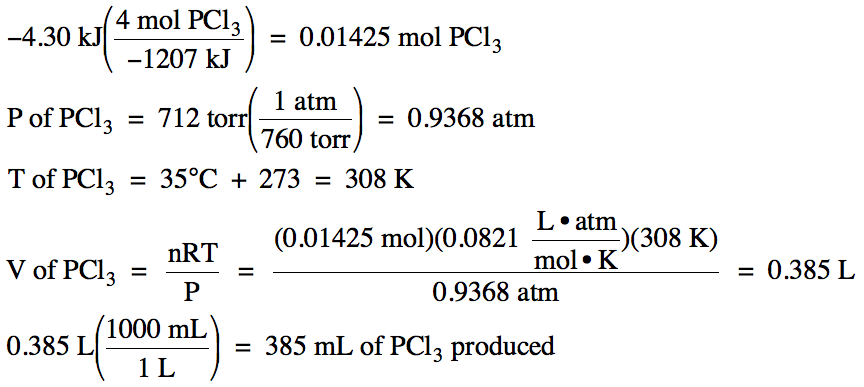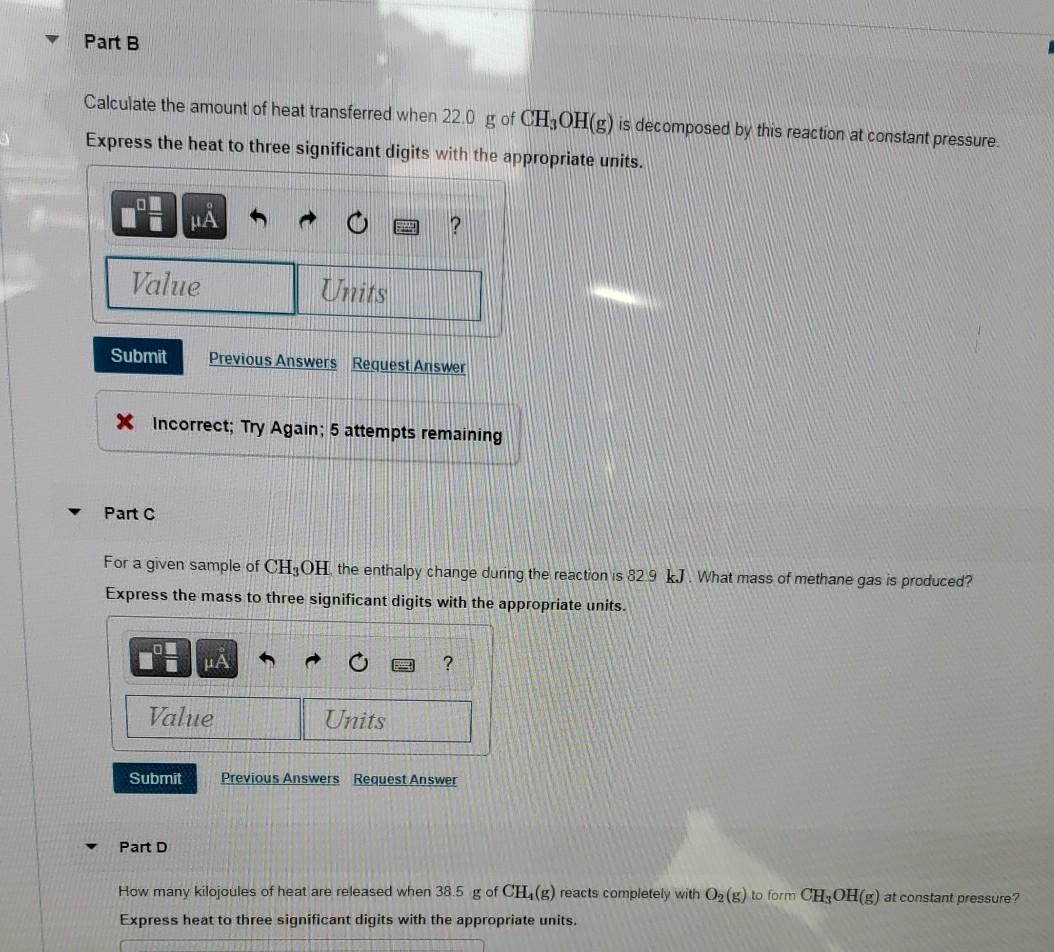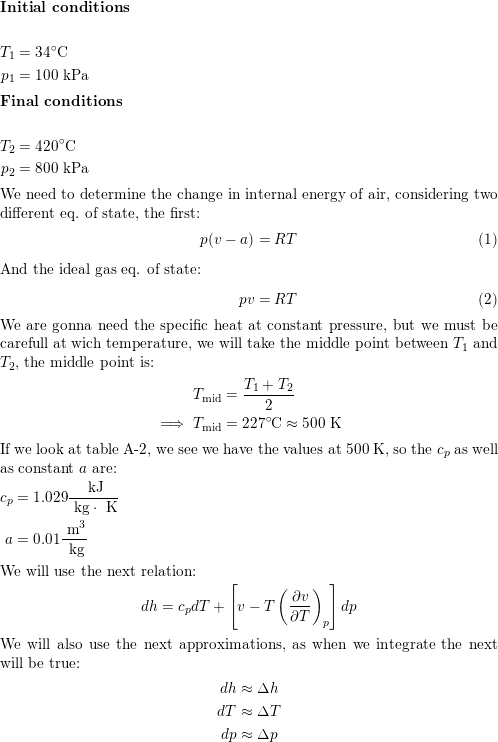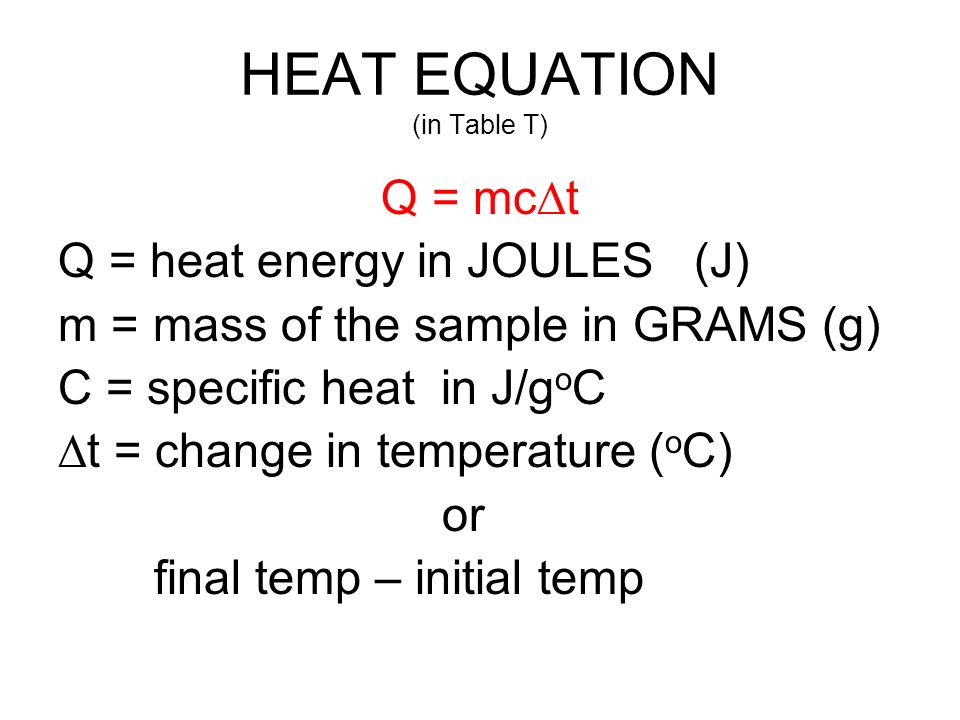
What is the temperature change in 224 g of water upon the absorption of 55 kJ of heat, the specific heat of water is 4.18 J/g °C? | Socratic

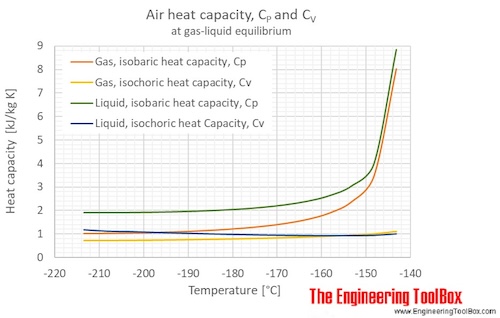
Specific Heat Capacity. Specific Heat Capacity (kJ/kg/°C) The specific heat is the amount of heat (measured in kJ) per unit mass (measured in Kg) required. - ppt download
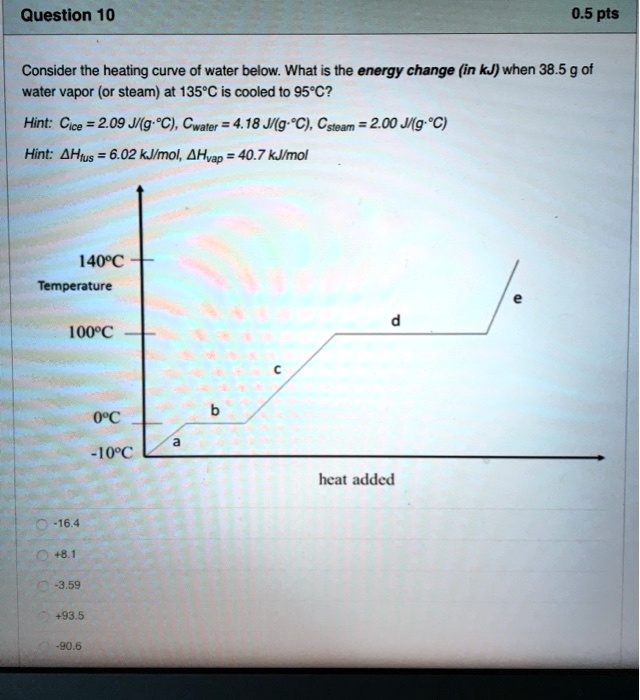
SOLVED: Question 10 0.5 pts Consider the heating curve of water below: What is the energy change (in kJ) when 38.5 9 ot water vapor (or steam) at 135*C is cooled to

USB C Tester,KJ-KayJI 2 in 1 Tester Color Screen IPS Digital Multimeter(2022),Voltage,Cur,Pwr,Resistance,Elec,Temp,Capacity,Tme,Fast Charging,with USB Clip Cable Support PD2.0/PD3.0,QC2.0/QC3.0,BC1.2: Amazon.com: Tools & Home Improvement
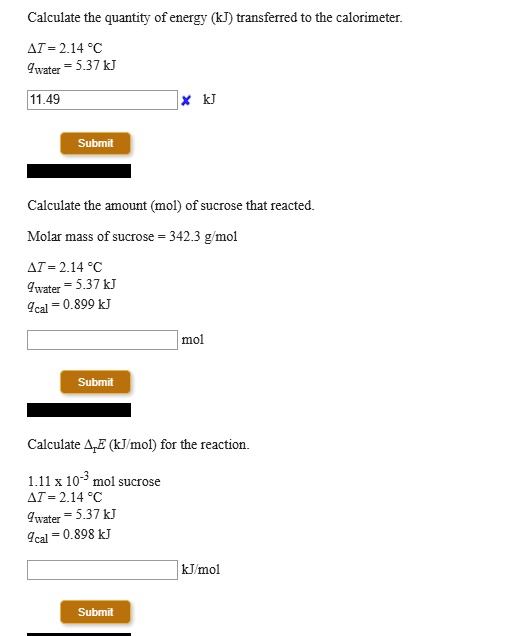
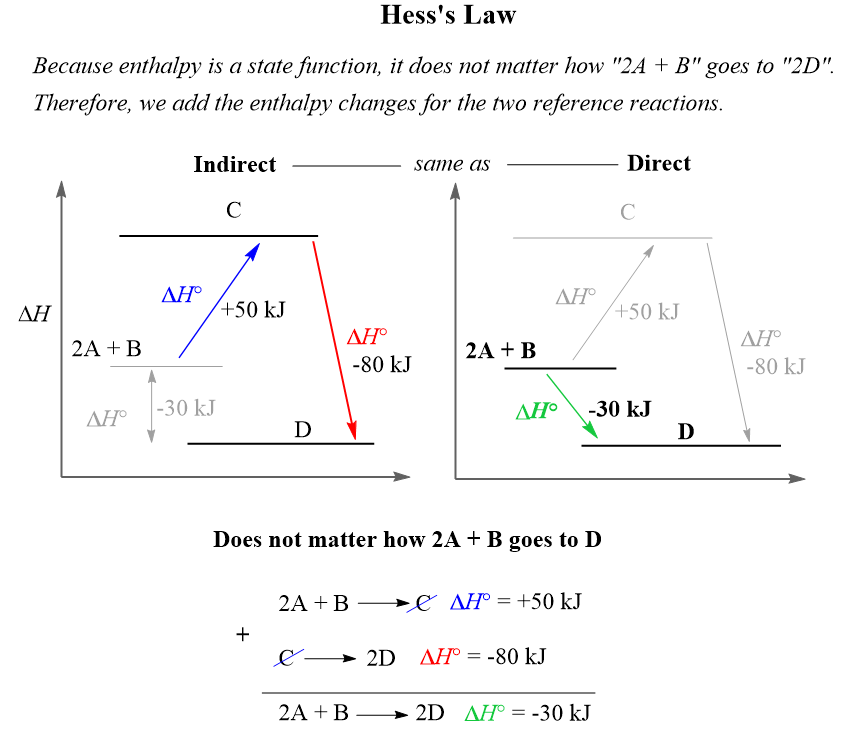
SOLVED: Calculate the quantity of energy (kJ) transferred to the calorimeter 1I=214"C Juzter 5337k 11.49 Submit Calculate the amount (mol) of sucrose that reacted Molar mass of sucrose 342.3 g mol 1T=214"C

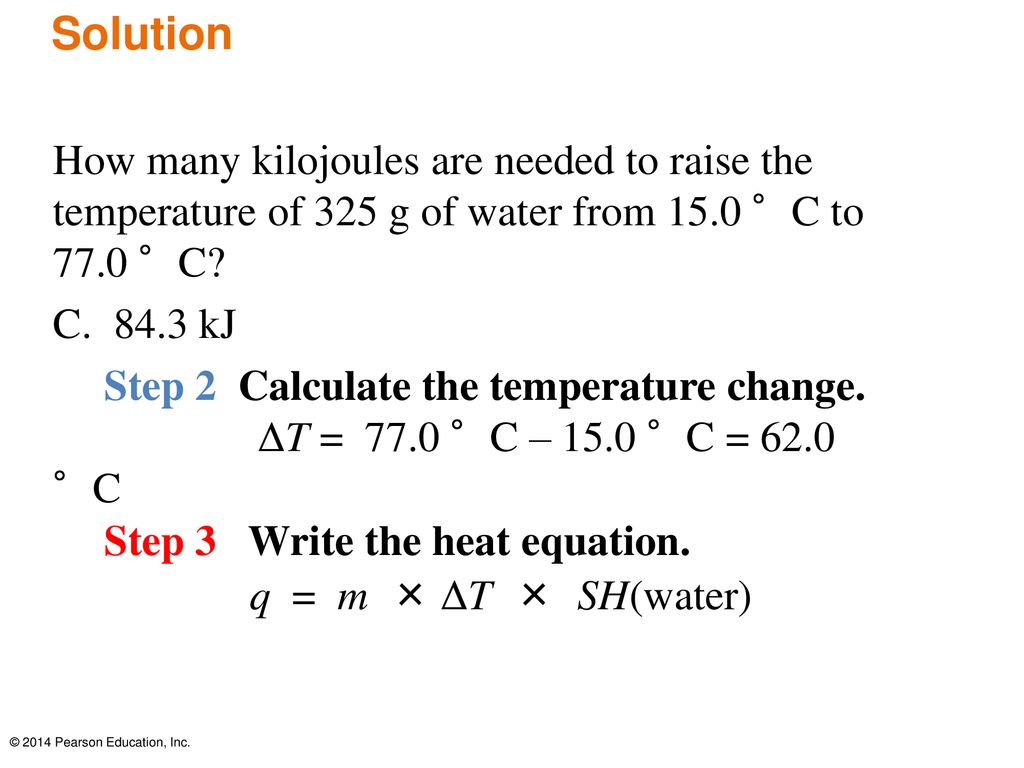
Calculate the number of KJ of heat necessary to raise the temperature of 60.0g of aluminium from.... - YouTube
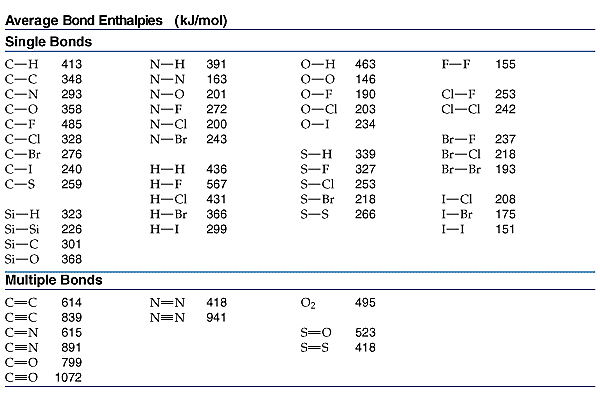
![Calculate resonance energy of C(6)H(6) (g). {:("Given :",Delta(f)[C(6)H(6)(g)]=-360 kJ mol^(-1)),(,DeltaH("Sub")[C("graphite")]=716 kJ mol^(-1)),(,B.E.(H-H)=437 kJ mol^(-1)),(,B.E.(C=C)=620 kJ mol^(-1)),(,B.E.(C-C)=340 kJ mol^(-1)),(,B.E.(C-H)=490 kJ ... Calculate resonance energy of C(6)H(6) (g). {:("Given :",Delta(f)[C(6)H(6)(g)]=-360 kJ mol^(-1)),(,DeltaH("Sub")[C("graphite")]=716 kJ mol^(-1)),(,B.E.(H-H)=437 kJ mol^(-1)),(,B.E.(C=C)=620 kJ mol^(-1)),(,B.E.(C-C)=340 kJ mol^(-1)),(,B.E.(C-H)=490 kJ ...](https://d10lpgp6xz60nq.cloudfront.net/web-thumb/32505248_web.png)
Calculate resonance energy of C(6)H(6) (g). {:("Given :",Delta(f)[C(6)H(6)(g)]=-360 kJ mol^(-1)),(,DeltaH("Sub")[C("graphite")]=716 kJ mol^(-1)),(,B.E.(H-H)=437 kJ mol^(-1)),(,B.E.(C=C)=620 kJ mol^(-1)),(,B.E.(C-C)=340 kJ mol^(-1)),(,B.E.(C-H)=490 kJ ...